ABSTRACT
Elbow injuries is a common injury seen in the acute setting. Radial head fractures are fairly common and accounts for approximately one third of elbow fractures1. Goals of management are directed at restoring early range of motion to prevent stiffness, pain and instability1. Post traumatic complications do occur infrequently but if missed can contribute to significant stiffness or instability symptoms. Heterotrophic ossification (HO) is a complication seen in elbow injury post dislocation, fracture, ligament, or tendon injuries. Excess lamellar bone infiltrates non osseous soft tissue with subsequent elbow stiffness. Radiographic imaging often will show irregular calcification that lack organization of mature cancellous or cortical bone2. Most papers cite the “Terrible triad” and Essex-Lopresti injury as a major contributor to HO. Terrible triad consists of radial head and coronoid process fractures with concurrent elbow dislocation3 4. Essex-Lopresti involves further injury of the distal radio-ulnar joint4. Nevertheless, there are other types of lesser injuries that can also cause HO. This paper focuses a bit on radial head fracture management, HO pathophysiology, and current available treatment options.
CASE HISTORY
Demographics: 53 year-old right hand dominant Samoan female was referred to fracture clinic by her general practitioner (GP) for further assessment and follow up.
Presenting Complaint: Presents with one week old un-displaced right radial head fracture.
History of Presenting Complaint: Injured right elbow by falling on outstretched hand. She developed immediate pain post fall.
Past Medical History: Obesity. Nil regular medications. No known drug allergies. Currently only taking paracetamol and ibuprofen as needed.
Examination: Reduced flexion/extension range of motion of right elbow from 30 to 120 degrees. Supination and pronation short by 10 degrees from full range of motion. No mechanical block. Upper limb neurology normal.
Investigation: Xray showed un-displaced right radial head fracture with elbow joint effusion.
Management: Rest in above elbow back-slab and sling for two weeks. Simple analgesia. At two weeks, she was referred to physiotherapy for early range of motion. She was monitored up to the three-month mark before discharging her from fracture clinic.
Clinical Question – Do I need to worry about HO in radial head/neck fractures?
DISCUSSION
The elbow joint articulation is made up of the humerus, proximal ulna and radial head. Together with the joint capsule, ligaments, tendons and muscles, the radial head is an important stabilizer of the elbow in valgus and supination4. Radial head fracture is a common injury accounting for a significant proportion of upper limb injuries. It is most commonly caused by axial load onto the radial head following a fall on an outstretched hand4. There are different types of radial head fractures. It was originally described by Mason in 1954 and was further modified by Hotchkiss in 19974. The current classification commonly used is the “Broberg-Morrey Classification”4. Table 1 below illustrates the types of radial head fractures classification according to their authors1.
Classification and Description of Radial Head Fractures | ||||||
Description of classification according to various authors | ||||||
Type | Mason | Johnston | Hotchkiss | Broberg- Morrey | Rineer | |
I | Without displacement | Without displacement | ˂2mm dislocation | ˂2mm dislocation | ||
II | With displacement | With displacement | ˃2 mm dislocation | ˃2-3 mm dislocation and involves ˃30% radial head | Cortical contact between fragments ˃stable No cortical contact between fragments ˃unstable | |
III | Comminuted | Comminuted | Comminuted | Comminuted | ||
IV | Comminuted | Fracture associated with dislocation of the elbow | Fracture associated with dislocation of the elbow | |||
Non displaced | Displaced stable | Displaced unstable | Comminuted | Fracture with elbow dislocation | ||
Treatment options can be conservative, or surgical which can involve open reduction internal fixation, e.g., screws, plates or even arthroplasty. The figure below shows a simplified version of the treatment algorithm in radial head fractures4.
In this paper, special interest is placed on HO post trauma/injury. HO is the formation of abnormal lamellar bone within non osseous soft tissues such as joint capsule, muscle, tendons, or ligaments3 5. Capsule contraction and adhesion remains the most common cause of post traumatic elbow stifness6. Anterior capsule contracture leads to flexion contracture and posterior capsule contracture causes extension contracture6. In the case of muscles, brachialis contracture limits extension and triceps contracture limits flexion movement6. HO can be genetically related or acquired. The main aetiologies for HO are: genetic predisposition, neurogenic from central nervous system injury; traumatic e.g., burns; or orthopaedic following a fracture, dislocation or surgical fixation3 7. Some literature has suggested that in radial head fracture, HO occurrence can be as high as 56%7. Studies have suggested no propensity of HO between genders or ethnic groups5.
The exact mechanism of HO is still a grey area. There is abundance of literature on HO over the years, albeit with significant heterogeneity in study methods3. HO leads to poor patient satisfaction and rehab progress. It leads to joint stiffness, oedema, reduced range of motion and ankylosis of joints3. The pathophysiology of HO is still not fully understood. Undifferentiated tissue respond to various signals which then stimulate the undifferentiated mesenchymal tissue to form fibroblast5. This fibroblast deposit collagen and extracellular matrix5. They form metaplastic tissue within the planes of HO which leads to subsequent formation of chondroblast and osteoblasts5. HO is also believed to be influenced by several factors such as prostaglandin E2, leukotrienes, bone morphogenic protein (BMP) and inflammatory process5. BMP production is stimulated by inflammation, venous stasis, and immobilization which then leads to increase in osteogenic cell differentiation5. HO typically occurs from one to three months post injury5. Xray can only pick up bone formation after 3-6 weeks of formation whereas bone scan is sensitive in recognizing bone metabolic activity as early as two weeks5.
Herman and colleagues performed systematic literature review on a few medical databases over a period of 26 years3. Strict inclusion and exclusion criteria were used to allow standardization of study selection. They also incorporated a grading system for HO i.e., Hastings and Graham classification system3. Anatomical sites looked at: distal humerus, proximal radius, proximal ulnar, terrible triad injury, elbow fracture dislocation and complex fracture3. The figure below illustrates the three grades of Hastings and Graham Classification3.
Class | Definition |
I | No functional impairment |
II | Subtotal functional impairment |
III | Ankylosis of joint |
Figure 2. Hastings and Graham Classification System
There is limited literature on non-steroidal anti-inflammatory (NSAID) prophylaxis for HO after elbow injury. The paper by Migliorini F. and colleagues on NSAIDs for prophylaxis for HO after total hip arthroplasty comes close to the clinical question8. This meta-analysis of randomized controlled trials looks at different NSAIDs and the rates of HO8. Celecoxib proved to be most efficacious in preventing HO although the authors acknowledged there are some limitations in their study which might confound their study results8. The other advantage gained from Celecoxib is the lower gastrointestinal side effect profile. Naproxen and Diclofenac also had some positive findings in HO prevention but not as convincing as Celecoxib8. The rest of the NSAIDs were statistically not significant. There were some limitations in the study i.e., some of studies were old, variability in study techniques and protocol heterogeneity8. On the other spectrum, NSAIDs have been notorious in delaying bone healing. NSAID’s initial role is inhibitory but long term effect is bone resorption by proliferation and formation of osteclasts2. The study by Chen and Dragoo reviewed the literature looking at non-selective and selective (COX-2) NSAIDs9. NSAID cause prostaglandin inhibition by acting on platelets, endothelial and mast cells9. They act mainly on the prostaglandin pathway involving cyclo-oxygenase, arachidonic acid, thromboxane and prostacyclin9. There are multiple pathways in tissue healing. Platelet derived growth factor (PDGF) is responsible for the mitogenic and chemotactic effects on other cells, e.g., smooth muscle cells, neutrophils, macrophages and fibroblasts9. The PDGF pathways remains unaffected by NSAIDs and the authors conclude that NSAIDs appears safe as other pathways are sufficient for bone healing9. Hence, the use of anti-inflammatories post sports injuries or surgeries is reasonable unless there are risk factors for poor bone healing9. Only Indomethacin appears to have deleterious effect in bone healing with no compelling evidence for the other NSAIDs9.
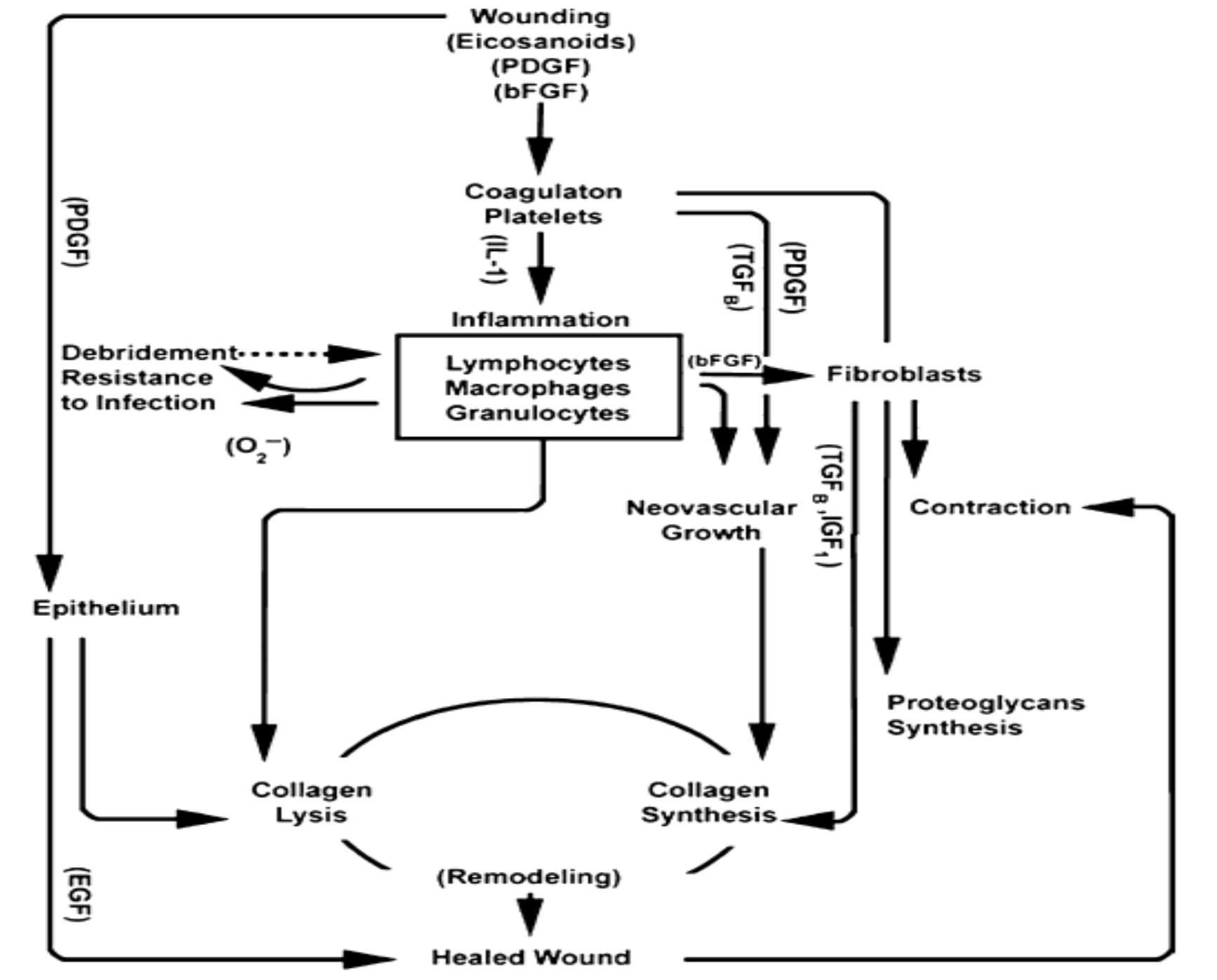
Herman ZJ. et al. suggested in their paper the incidence of HO after surgery was as high at 28.7%3. They further concluded that rates of HO was higher in injuries such as proximal humerus fractures, “terrible triad” and elbow fractures compared to distal humerus fractures3. One useful treatment option for post traumatic elbow stiffness is arthroscopic arthrolysis. Dai J et al. did a retrospective review of 30 arthroscopic elbow releases6. There was an improvement in the elbow range of motion post operatively6. The paper had some weakness, i.e., it was a non-randomised retrospective study with a short term follow up of 30 patients. There was no treatment comparison group. Although, the results were good post op, it was not convincing enough. The author also further elucidates the importance of recognizing which soft tissue structures are affected to aid good outcome6.
To date, there is no goal standard effective treatment option for HO. There has been hypothesis on the use of radiotherapy based on the role of osteoprogenitor cells7. Radiation is used to suppress the pluripotent mesenchymal cells from proliferating and differentiating to osteoprogenitor and osteoblast7. Bone morphogenic protein (BMP) -7 is suppressed which plays a role in the formal of mature bone tissue from osteoblast7. Albeit there is no “gold standard” radiotherapy treatment regimen due to the paucity of literature. The amount of dose, timing and appropriate indication remains mainly anecdotal evidence7. The systematic review of literature by paper by Ploumis A. and colleagues looked at radiotherapy for HO prevention7. Not surprising, out of the 27 studies, only one is a randomized trial and the rest being case reports and series7. Radiotherapy was not statistically significant with similar outcomes to NSAID use for prevention of HO. Moreover, radiotherapy had a list of adverse effects such as skin thinning, ulceration, poor wound healing, delayed/non-union, superimposed infection and risk of carcinogenesis7. Therefore, radiotherapy is not a first line or safe intervention for the prevention of elbow HO7.
CONCLUSION
There are multiple studies out there looking at HO prevention but the most effective treatment remains elusive8. Society’s paradigm of the ideal medical treatment for HO is vexatious. Failure to treatment can often lead to patient and clinician frustration. The notion of every medical condition has an easy treatment is an erroneous assumption. HO pathophysiology is still not fully understood. Invariably, for treatment to be successful, the pathophysiology of the condition must be well understood. As new treatment strategies evolved, there is abundance of literature to provide an overview of HO and an effective treatment option1. Coming back to my clinical question, we should follow up radial head or elbow injuries up to at least three months to identify HO. Early recognition of HO might lead to better outcomes. Patients can then be offered different treatment options.
Reference
- Kodde IF, Kaas L, Flipsen M, et al. Current concepts in the management of radial head fractures. World journal of orthopedics 2015;6(11):954.
- Buckwalter J, Glimcher M, Cooper R, et al. Bone biology. II: Formation, form, modeling, remodeling, and regulation of cell function. Journal of bone and joint surgery American volume 1995;77(8):1276-89.
- Herman ZJ, Edelman DG, Ilyas AM. Heterotopic ossification after elbow fractures. Orthopedics 2021;44(1):10-16.
- Khawar H, Craxford S, Ollivere B. Radial head fractures. British Journal of Hospital Medicine 2020;81(4):1-6.
- Zychowicz ME. Pathophysiology of Heterotopic Ossification. Orthopaedic Nursing 2013;32(3):173-77. doi: 10.1097/NOR.0b013e3182920d85
- Dai J, Zhang G, Li S, et al. Arthroscopic Treatment of Posttraumatic Elbow Stiffness Due to Soft Tissue Problems. Orthopaedic Surgery 2020;12(5):1464-70.
- Ploumis A, Belbasis L, Ntzani E, et al. Radiotherapy for prevention of heterotopic ossification of the elbow: a systematic review of the literature. Journal of shoulder and elbow surgery 2013;22(11):1580-88.
- Migliorini F, Trivellas A, Eschweiler J, et al. NSAIDs for Prophylaxis for Heterotopic Ossification After Total Hip Arthroplasty: A Bayesian Network Meta-analysis. Calcified Tissue International 2020:1-11.
- Chen MR, Dragoo JL. The effect of nonsteroidal anti-inflammatory drugs on tissue healing. Knee Surgery, Sports Traumatology, Arthroscopy 2013;21(3):540-49.
